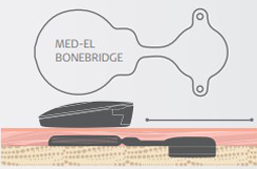
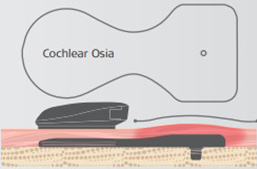
The Sensible Choice
MED-EL built BONEBRIDGE on over a decade of experience from recipients around the world. Why trust your patients' hearing to anyone else? We know what it takes to make a dependable bone conduction implant system. See for yourself how MED-EL BONEBRIDGE is the sensible choice.
Available
Not available
1. BONEBRIDGE was first approved in April 2012 (CE-Mark BCI 601), Osia was first approved in July 2019 (FDA Clearance Osia 1).
2. MED-EL Corporation. (2023). BONEBRIDGE BCI 602 Implant Kit: Instructions for use. MED-EL Elektromedizinische Geräte GmbH.
3. ©Cochlear Limited. (2024). Cochlear™ Osia® OSI300 Implant Physician’s Guide. Cochlear Group.
4. MED-EL Corporation. (2023). BONEBRIDGE SAMBA 2 Audio Processor: Instructions for use. MED-EL Elektromedizinische Geräte GmbH.
5. ©Cochlear Limited. (2023). Datasheet Cochlear™ Osia® System. Cochlear Group.
6. ©Cochlear Limited. (2023). Cochlear™ Osia® Magnetic Resonance Imaging (MRI) Guidelines. Cochlear Group.
*Osia is a trademark of Cochlear Ltd, Australia.
†BONEBRIDGE is MR conditional at 1.5 Tesla following the conditions detailed here: medel.com/important-safety-information.
Are you ready to get your hands on BONEBRIDGE?
See it in action and experience the SMART DESIGN for yourself.